A review on Fenton-like processes for organic wastewater treatment
1
2016
... Fenton法作为一种高级氧化工艺(AOP)因具有操作模式简单、适用范围广泛以及氧化能力强等特点,广泛应用于污水处理中〔1〕.传统的Fenton反应主要是用亚铁离子(Fe2+)催化分解过氧化氢(H2O2)产生的羟基自由基(·OH)降解有机物.·OH是氧化能力仅次于氟的氧化剂,氧化电位为2.8 eV,具有高反应性和非选择性,几乎可以将任何有机物氧化为无机离子和CO2〔2〕.Fenton反应主要方程式如下〔3〕: ...
Treatment of municipal wastewater treatment plant effluents with modified photo-Fenton as a tertiary treatment for the degradation of micro pollutants and disinfection
1
2012
... Fenton法作为一种高级氧化工艺(AOP)因具有操作模式简单、适用范围广泛以及氧化能力强等特点,广泛应用于污水处理中〔1〕.传统的Fenton反应主要是用亚铁离子(Fe2+)催化分解过氧化氢(H2O2)产生的羟基自由基(·OH)降解有机物.·OH是氧化能力仅次于氟的氧化剂,氧化电位为2.8 eV,具有高反应性和非选择性,几乎可以将任何有机物氧化为无机离子和CO2〔2〕.Fenton反应主要方程式如下〔3〕: ...
Arsenic (Ⅲ) and iron(Ⅱ) co-oxidation by oxygen and hydrogen peroxide: Divergent reactions in the presence of organic ligands
1
2013
... Fenton法作为一种高级氧化工艺(AOP)因具有操作模式简单、适用范围广泛以及氧化能力强等特点,广泛应用于污水处理中〔1〕.传统的Fenton反应主要是用亚铁离子(Fe2+)催化分解过氧化氢(H2O2)产生的羟基自由基(·OH)降解有机物.·OH是氧化能力仅次于氟的氧化剂,氧化电位为2.8 eV,具有高反应性和非选择性,几乎可以将任何有机物氧化为无机离子和CO2〔2〕.Fenton反应主要方程式如下〔3〕: ...
Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes
1
2014
... 传统的Fenton反应只能在pH较低的酸性环境下进行,pH约为3.0~4.0〔4〕,pH > 4.0会显著降低污染物的去除率〔5〕.当pH > 4.0时,Fe3+将以不溶性络合物的形式存在,会极大地影响式(2)中Fe3+的还原,使得Fenton反应无法进行.因此,采用Fenton法对污水进行处理,往往需要增加酸化预处理,这不但增加了处理成本,而且在含有硫化物或氰化物的污水中,酸化会导致某些有毒有害气体释放到环境中〔6〕.对传统的Fenton法进行改良,使其能够在宽泛的pH下进行,将会极大提升Fenton氧化技术在废水处理中的应用前景. ...
Inorganic chelated modified-Fenton treatment of polycyclic aromatic hydrocarbon(PAH)-contaminated soils
1
2012
... 传统的Fenton反应只能在pH较低的酸性环境下进行,pH约为3.0~4.0〔4〕,pH > 4.0会显著降低污染物的去除率〔5〕.当pH > 4.0时,Fe3+将以不溶性络合物的形式存在,会极大地影响式(2)中Fe3+的还原,使得Fenton反应无法进行.因此,采用Fenton法对污水进行处理,往往需要增加酸化预处理,这不但增加了处理成本,而且在含有硫化物或氰化物的污水中,酸化会导致某些有毒有害气体释放到环境中〔6〕.对传统的Fenton法进行改良,使其能够在宽泛的pH下进行,将会极大提升Fenton氧化技术在废水处理中的应用前景. ...
Effect of humic substances on the Fenton treatment of wastewater at acidic and neutral pH
1
2008
... 传统的Fenton反应只能在pH较低的酸性环境下进行,pH约为3.0~4.0〔4〕,pH > 4.0会显著降低污染物的去除率〔5〕.当pH > 4.0时,Fe3+将以不溶性络合物的形式存在,会极大地影响式(2)中Fe3+的还原,使得Fenton反应无法进行.因此,采用Fenton法对污水进行处理,往往需要增加酸化预处理,这不但增加了处理成本,而且在含有硫化物或氰化物的污水中,酸化会导致某些有毒有害气体释放到环境中〔6〕.对传统的Fenton法进行改良,使其能够在宽泛的pH下进行,将会极大提升Fenton氧化技术在废水处理中的应用前景. ...
Preparation of magnetitebased catalysts and their application in heterogeneous Fenton oxidation: A review
2
2015
... 矿物金属催化剂是最常见的能拓宽Fenton反应适用范围的催化剂,其在宽泛的pH下能展示出高效的催化活性〔7〕.矿物金属催化剂中用作Fenton反应的催化剂以铁基催化剂为主,包括针铁矿〔8〕、磁铁矿〔9〕、菱铁矿〔10〕、黄铁矿〔11〕等.在铁基催化剂参与的Fenton体系中,H2O2首先将氧化铁表面的Fe(Ⅲ)还原成Fe(Ⅱ),溶液中的Fe(Ⅱ)再催化H2O2生成·OH,从而有效降解有机污染物〔12〕. ...
... 铁基催化剂不仅可以直接投入使用,更多的是将其改良成负载型催化剂,以避免在与目标物接触之前发生聚合作用,导致催化活性下降〔7〕.改良后的催化剂活性更强,能多次重复利用,可更广泛更高效地氧化降解污染物.常见的用于Fenton反应的负载型铁基催化剂如表 1所示. ...
Effects of low molecular weight organic acids and fulvic acid on 2, 4, 4'-trichlorobiphenyl degradation and hydroxyl radical formation in a goethite-catalyzed Fentonlike reaction
1
2017
... 矿物金属催化剂是最常见的能拓宽Fenton反应适用范围的催化剂,其在宽泛的pH下能展示出高效的催化活性〔7〕.矿物金属催化剂中用作Fenton反应的催化剂以铁基催化剂为主,包括针铁矿〔8〕、磁铁矿〔9〕、菱铁矿〔10〕、黄铁矿〔11〕等.在铁基催化剂参与的Fenton体系中,H2O2首先将氧化铁表面的Fe(Ⅲ)还原成Fe(Ⅱ),溶液中的Fe(Ⅱ)再催化H2O2生成·OH,从而有效降解有机污染物〔12〕. ...
Heterogeneous UV/Fenton degradation of TBBPA catalyzed by titanomagnetite: Catalyst characterization, performance and degradation products
1
2012
... 矿物金属催化剂是最常见的能拓宽Fenton反应适用范围的催化剂,其在宽泛的pH下能展示出高效的催化活性〔7〕.矿物金属催化剂中用作Fenton反应的催化剂以铁基催化剂为主,包括针铁矿〔8〕、磁铁矿〔9〕、菱铁矿〔10〕、黄铁矿〔11〕等.在铁基催化剂参与的Fenton体系中,H2O2首先将氧化铁表面的Fe(Ⅲ)还原成Fe(Ⅱ),溶液中的Fe(Ⅱ)再催化H2O2生成·OH,从而有效降解有机污染物〔12〕. ...
A novel discovery of a heterogeneous Fenton-like system based on natural siderite: A wide range of pH values from 3 to 9
2
2020
... 矿物金属催化剂是最常见的能拓宽Fenton反应适用范围的催化剂,其在宽泛的pH下能展示出高效的催化活性〔7〕.矿物金属催化剂中用作Fenton反应的催化剂以铁基催化剂为主,包括针铁矿〔8〕、磁铁矿〔9〕、菱铁矿〔10〕、黄铁矿〔11〕等.在铁基催化剂参与的Fenton体系中,H2O2首先将氧化铁表面的Fe(Ⅲ)还原成Fe(Ⅱ),溶液中的Fe(Ⅱ)再催化H2O2生成·OH,从而有效降解有机污染物〔12〕. ...
... Fuwei Sun等〔10〕选择天然菱铁矿作为Fenton催化剂催化降解水中的抗生素磺胺嘧啶钠,该研究将6 g/L菱铁矿和100 mmol/L H2O2加入到10 mg/L的磺胺嘧啶钠溶液中.结果表明,在溶液pH为3~9的范围内,均显示出优异的降解效果,3 h内降解率达到98%.Zhirong Lin等〔13〕研究了不同pH条件下以针铁矿作为催化剂的类Fenton体系降解水中2,4,4’-三氯联苯(PCB28)的效率.其在1 mg/L的PCB28溶液中,加入1 g/L的针铁矿和3.4 g/L的H2O2.结果表明,当pH为3时,反应48 h后PCB28降解率达到99%;pH为7时,PCB28的降解率仍能达到52%.可以通过提高针铁矿的量来增加针铁矿表面上的活性位点,从而提升降解率. ...
Enhanced degradation of chloramphenicol at alkaline conditions by S(-Ⅱ) assisted heterogeneous Fenton-like reactions using pyrite
1
2017
... 矿物金属催化剂是最常见的能拓宽Fenton反应适用范围的催化剂,其在宽泛的pH下能展示出高效的催化活性〔7〕.矿物金属催化剂中用作Fenton反应的催化剂以铁基催化剂为主,包括针铁矿〔8〕、磁铁矿〔9〕、菱铁矿〔10〕、黄铁矿〔11〕等.在铁基催化剂参与的Fenton体系中,H2O2首先将氧化铁表面的Fe(Ⅲ)还原成Fe(Ⅱ),溶液中的Fe(Ⅱ)再催化H2O2生成·OH,从而有效降解有机污染物〔12〕. ...
Clays and oxide minerals as catalysts and nanocatalysts in Fenton-like reactions: A review
1
2010
... 矿物金属催化剂是最常见的能拓宽Fenton反应适用范围的催化剂,其在宽泛的pH下能展示出高效的催化活性〔7〕.矿物金属催化剂中用作Fenton反应的催化剂以铁基催化剂为主,包括针铁矿〔8〕、磁铁矿〔9〕、菱铁矿〔10〕、黄铁矿〔11〕等.在铁基催化剂参与的Fenton体系中,H2O2首先将氧化铁表面的Fe(Ⅲ)还原成Fe(Ⅱ),溶液中的Fe(Ⅱ)再催化H2O2生成·OH,从而有效降解有机污染物〔12〕. ...
Kinetics and products of PCB28 degradation through a goethite-catalyzed Fenton-like reaction
1
2014
... Fuwei Sun等〔10〕选择天然菱铁矿作为Fenton催化剂催化降解水中的抗生素磺胺嘧啶钠,该研究将6 g/L菱铁矿和100 mmol/L H2O2加入到10 mg/L的磺胺嘧啶钠溶液中.结果表明,在溶液pH为3~9的范围内,均显示出优异的降解效果,3 h内降解率达到98%.Zhirong Lin等〔13〕研究了不同pH条件下以针铁矿作为催化剂的类Fenton体系降解水中2,4,4’-三氯联苯(PCB28)的效率.其在1 mg/L的PCB28溶液中,加入1 g/L的针铁矿和3.4 g/L的H2O2.结果表明,当pH为3时,反应48 h后PCB28降解率达到99%;pH为7时,PCB28的降解率仍能达到52%.可以通过提高针铁矿的量来增加针铁矿表面上的活性位点,从而提升降解率. ...
Assessment of iron chelates efficiency for photo-Fenton at neutral pH
1
2014
... 矿物金属催化剂的使用能够解决Fenton反应pH范围狭窄的缺陷,但催化剂的催化活性仍然有待提高.引入螯合剂能有效地提高催化剂的催化活性.常见的螯合剂有乙二胺四乙酸(EDTA)〔14〕、乙二胺二琥珀酸三钠(EDDS)〔15〕、次氮基三乙酸(NTA)〔16〕、柠檬酸盐〔17〕、酒石酸〔18〕等.Shengpeng Sun等〔19〕在Fe3O4催化降解卡马西平(CBZ)的Fenton反应体系中引入了NTA.结果表明,在6.35×10-2 mmol/L的CBZ溶液中,加入1.0 g/L的Fe3O4,100 mmol/L的H2O2和0.5 mmol/L的NTA,反应120 min后,CBZ去除率为99.6%;而未添加NTA的对照组,仅有6%的CBZ被去除.Hongwei Sun等〔20〕建立了具有抗坏血酸(AA)、磁铁矿(Fe3O4)和H2O2的Fenton反应体系,并用其降解水中甲草胺.结果表明,在一定的试验条件下,20 min内甲草胺去除率就可以达到69.5%;而不含AA的Fenton体系,1 h内对甲草胺的去除率不足10%.这证明了AA能够促进Fenton反应降解甲草胺. ...
Removal of pharmaceuticals from MWTP effluent by nanofiltration and solar photoFenton using two different iron complexes at neutral pH
1
2014
... 矿物金属催化剂的使用能够解决Fenton反应pH范围狭窄的缺陷,但催化剂的催化活性仍然有待提高.引入螯合剂能有效地提高催化剂的催化活性.常见的螯合剂有乙二胺四乙酸(EDTA)〔14〕、乙二胺二琥珀酸三钠(EDDS)〔15〕、次氮基三乙酸(NTA)〔16〕、柠檬酸盐〔17〕、酒石酸〔18〕等.Shengpeng Sun等〔19〕在Fe3O4催化降解卡马西平(CBZ)的Fenton反应体系中引入了NTA.结果表明,在6.35×10-2 mmol/L的CBZ溶液中,加入1.0 g/L的Fe3O4,100 mmol/L的H2O2和0.5 mmol/L的NTA,反应120 min后,CBZ去除率为99.6%;而未添加NTA的对照组,仅有6%的CBZ被去除.Hongwei Sun等〔20〕建立了具有抗坏血酸(AA)、磁铁矿(Fe3O4)和H2O2的Fenton反应体系,并用其降解水中甲草胺.结果表明,在一定的试验条件下,20 min内甲草胺去除率就可以达到69.5%;而不含AA的Fenton体系,1 h内对甲草胺的去除率不足10%.这证明了AA能够促进Fenton反应降解甲草胺. ...
Performance evaluation of different air distribution systems in an aircraft cabin mockup
1
2017
... 矿物金属催化剂的使用能够解决Fenton反应pH范围狭窄的缺陷,但催化剂的催化活性仍然有待提高.引入螯合剂能有效地提高催化剂的催化活性.常见的螯合剂有乙二胺四乙酸(EDTA)〔14〕、乙二胺二琥珀酸三钠(EDDS)〔15〕、次氮基三乙酸(NTA)〔16〕、柠檬酸盐〔17〕、酒石酸〔18〕等.Shengpeng Sun等〔19〕在Fe3O4催化降解卡马西平(CBZ)的Fenton反应体系中引入了NTA.结果表明,在6.35×10-2 mmol/L的CBZ溶液中,加入1.0 g/L的Fe3O4,100 mmol/L的H2O2和0.5 mmol/L的NTA,反应120 min后,CBZ去除率为99.6%;而未添加NTA的对照组,仅有6%的CBZ被去除.Hongwei Sun等〔20〕建立了具有抗坏血酸(AA)、磁铁矿(Fe3O4)和H2O2的Fenton反应体系,并用其降解水中甲草胺.结果表明,在一定的试验条件下,20 min内甲草胺去除率就可以达到69.5%;而不含AA的Fenton体系,1 h内对甲草胺的去除率不足10%.这证明了AA能够促进Fenton反应降解甲草胺. ...
Bacterial inactivation with iron citrate complex: A new source of dissolved iron in solar photo-Fenton process at near-neutral and alkaline pH
1
2016
... 矿物金属催化剂的使用能够解决Fenton反应pH范围狭窄的缺陷,但催化剂的催化活性仍然有待提高.引入螯合剂能有效地提高催化剂的催化活性.常见的螯合剂有乙二胺四乙酸(EDTA)〔14〕、乙二胺二琥珀酸三钠(EDDS)〔15〕、次氮基三乙酸(NTA)〔16〕、柠檬酸盐〔17〕、酒石酸〔18〕等.Shengpeng Sun等〔19〕在Fe3O4催化降解卡马西平(CBZ)的Fenton反应体系中引入了NTA.结果表明,在6.35×10-2 mmol/L的CBZ溶液中,加入1.0 g/L的Fe3O4,100 mmol/L的H2O2和0.5 mmol/L的NTA,反应120 min后,CBZ去除率为99.6%;而未添加NTA的对照组,仅有6%的CBZ被去除.Hongwei Sun等〔20〕建立了具有抗坏血酸(AA)、磁铁矿(Fe3O4)和H2O2的Fenton反应体系,并用其降解水中甲草胺.结果表明,在一定的试验条件下,20 min内甲草胺去除率就可以达到69.5%;而不含AA的Fenton体系,1 h内对甲草胺的去除率不足10%.这证明了AA能够促进Fenton反应降解甲草胺. ...
Remarkable enhancement of bacterial inactivation in wastewater through promotion of solar photo-Fenton at near-neutral pH by natural organic acids
1
2017
... 矿物金属催化剂的使用能够解决Fenton反应pH范围狭窄的缺陷,但催化剂的催化活性仍然有待提高.引入螯合剂能有效地提高催化剂的催化活性.常见的螯合剂有乙二胺四乙酸(EDTA)〔14〕、乙二胺二琥珀酸三钠(EDDS)〔15〕、次氮基三乙酸(NTA)〔16〕、柠檬酸盐〔17〕、酒石酸〔18〕等.Shengpeng Sun等〔19〕在Fe3O4催化降解卡马西平(CBZ)的Fenton反应体系中引入了NTA.结果表明,在6.35×10-2 mmol/L的CBZ溶液中,加入1.0 g/L的Fe3O4,100 mmol/L的H2O2和0.5 mmol/L的NTA,反应120 min后,CBZ去除率为99.6%;而未添加NTA的对照组,仅有6%的CBZ被去除.Hongwei Sun等〔20〕建立了具有抗坏血酸(AA)、磁铁矿(Fe3O4)和H2O2的Fenton反应体系,并用其降解水中甲草胺.结果表明,在一定的试验条件下,20 min内甲草胺去除率就可以达到69.5%;而不含AA的Fenton体系,1 h内对甲草胺的去除率不足10%.这证明了AA能够促进Fenton反应降解甲草胺. ...
Enhanced heterogeneous and homogeneous Fenton-like degradation of carbamazepine by nano-Fe3O4/H2O2 with nitrilotriacetic acid
1
2014
... 矿物金属催化剂的使用能够解决Fenton反应pH范围狭窄的缺陷,但催化剂的催化活性仍然有待提高.引入螯合剂能有效地提高催化剂的催化活性.常见的螯合剂有乙二胺四乙酸(EDTA)〔14〕、乙二胺二琥珀酸三钠(EDDS)〔15〕、次氮基三乙酸(NTA)〔16〕、柠檬酸盐〔17〕、酒石酸〔18〕等.Shengpeng Sun等〔19〕在Fe3O4催化降解卡马西平(CBZ)的Fenton反应体系中引入了NTA.结果表明,在6.35×10-2 mmol/L的CBZ溶液中,加入1.0 g/L的Fe3O4,100 mmol/L的H2O2和0.5 mmol/L的NTA,反应120 min后,CBZ去除率为99.6%;而未添加NTA的对照组,仅有6%的CBZ被去除.Hongwei Sun等〔20〕建立了具有抗坏血酸(AA)、磁铁矿(Fe3O4)和H2O2的Fenton反应体系,并用其降解水中甲草胺.结果表明,在一定的试验条件下,20 min内甲草胺去除率就可以达到69.5%;而不含AA的Fenton体系,1 h内对甲草胺的去除率不足10%.这证明了AA能够促进Fenton反应降解甲草胺. ...
Ascorbic acid promoted magnetite Fenton degradation of alachlor: Mechanistic insights and kinetic modeling
1
2020
... 矿物金属催化剂的使用能够解决Fenton反应pH范围狭窄的缺陷,但催化剂的催化活性仍然有待提高.引入螯合剂能有效地提高催化剂的催化活性.常见的螯合剂有乙二胺四乙酸(EDTA)〔14〕、乙二胺二琥珀酸三钠(EDDS)〔15〕、次氮基三乙酸(NTA)〔16〕、柠檬酸盐〔17〕、酒石酸〔18〕等.Shengpeng Sun等〔19〕在Fe3O4催化降解卡马西平(CBZ)的Fenton反应体系中引入了NTA.结果表明,在6.35×10-2 mmol/L的CBZ溶液中,加入1.0 g/L的Fe3O4,100 mmol/L的H2O2和0.5 mmol/L的NTA,反应120 min后,CBZ去除率为99.6%;而未添加NTA的对照组,仅有6%的CBZ被去除.Hongwei Sun等〔20〕建立了具有抗坏血酸(AA)、磁铁矿(Fe3O4)和H2O2的Fenton反应体系,并用其降解水中甲草胺.结果表明,在一定的试验条件下,20 min内甲草胺去除率就可以达到69.5%;而不含AA的Fenton体系,1 h内对甲草胺的去除率不足10%.这证明了AA能够促进Fenton反应降解甲草胺. ...
Decomposition of 2-chlorophenol employing goethite as Fenton catalyst.Ⅰ. Proposal of a feasible, combined reaction scheme of heterogeneous and homogeneous reactions
1
2010
... 矿物铁基催化剂成本较低,制备相对简单,但直接投用受环境影响很大,H2O2利用效率不高.通过添加螯合剂,能进一步提升高pH下该类催化剂的催化活性.在Fenton反应中引入某些螯合剂,能从2个方面提高铁基催化剂的催化活性:(1)能够加快铁的浸出〔21〕,并在高pH下与Fe(Ⅲ)/ Fe(Ⅱ)形成络合物,使其保持可溶状态,使溶液中的均相Fenton反应得以继续;(2)能够提高矿物催化剂表面Fe(Ⅲ)/ Fe(Ⅱ)的循环,加快表面非均相Fenton反应.催化剂的活性主要与这些螯合物的稳定性、官能团种类、自由基清除能力有关〔22〕. ...
Homogeneous photo-Fenton processes at near neutral pH: A review
1
2017
... 矿物铁基催化剂成本较低,制备相对简单,但直接投用受环境影响很大,H2O2利用效率不高.通过添加螯合剂,能进一步提升高pH下该类催化剂的催化活性.在Fenton反应中引入某些螯合剂,能从2个方面提高铁基催化剂的催化活性:(1)能够加快铁的浸出〔21〕,并在高pH下与Fe(Ⅲ)/ Fe(Ⅱ)形成络合物,使其保持可溶状态,使溶液中的均相Fenton反应得以继续;(2)能够提高矿物催化剂表面Fe(Ⅲ)/ Fe(Ⅱ)的循环,加快表面非均相Fenton反应.催化剂的活性主要与这些螯合物的稳定性、官能团种类、自由基清除能力有关〔22〕. ...
Carbonaceous-TiO2 nanomaterials for photocatalytic degradation of pollutants: A review
1
2017
... 催化剂的负载材料分为2类:(1)碳基材料.主要有石墨碳、石墨碳氮化物、石墨烯、生物炭、活性炭等材料.对于非均相Fenton反应而言,这些材料具有高稳定性,结构可调,并具有一定的化学惰性和良好的电性能〔23〕,是改善金属催化剂活性的关键,已经成为目前主流的负载材料;(2)非碳基材料.主要有硅藻土〔24〕、黏土〔25〕、凝胶等材料.这些材料能增加反应位点,减少催化剂浸出量,且材料本身对环境十分友好,不会给环境带来新的污染. ...
Residual diatomaceous earth as a potential and cost effective biosorbent of the azo textile dye Reactive Blue 160
1
2020
... 催化剂的负载材料分为2类:(1)碳基材料.主要有石墨碳、石墨碳氮化物、石墨烯、生物炭、活性炭等材料.对于非均相Fenton反应而言,这些材料具有高稳定性,结构可调,并具有一定的化学惰性和良好的电性能〔23〕,是改善金属催化剂活性的关键,已经成为目前主流的负载材料;(2)非碳基材料.主要有硅藻土〔24〕、黏土〔25〕、凝胶等材料.这些材料能增加反应位点,减少催化剂浸出量,且材料本身对环境十分友好,不会给环境带来新的污染. ...
Fenton-like processes and adsorption using iron oxide-pillared clay with magnetic properties for organic compound mitigation
1
2015
... 催化剂的负载材料分为2类:(1)碳基材料.主要有石墨碳、石墨碳氮化物、石墨烯、生物炭、活性炭等材料.对于非均相Fenton反应而言,这些材料具有高稳定性,结构可调,并具有一定的化学惰性和良好的电性能〔23〕,是改善金属催化剂活性的关键,已经成为目前主流的负载材料;(2)非碳基材料.主要有硅藻土〔24〕、黏土〔25〕、凝胶等材料.这些材料能增加反应位点,减少催化剂浸出量,且材料本身对环境十分友好,不会给环境带来新的污染. ...
Evaluation of MnO2-templated iron oxide-coated diatomites for their catalytic performance in heterogeneous photo Fenton-like system
1
2018
... 常见的负载铁基催化剂在Fenton反应中的应用效果
| 催化剂 | 催化剂支撑材料 | 降解目标物 | 最优结果 | 催化剂稳定性 | 适用pH范围 | 参考文献 |
| α-Fe2O3 | 硅藻土 | 亚甲基蓝(MB) | 120 min后去除率达到98% | 重复使用5次,稳定性良好 | 在pH为2.0~8.0范围内去除效果良好 | 〔26〕 |
| α-Fe2O3 | 石墨相碳氮化物(g-C3N4) | 四环素(TC) | 60 min后去除率为95% | 重复使用5次,稳定性良好 | 在pH为2.01~9.09范围内去除效果良好 | 〔27〕 |
| γ-Fe2O3 | 石墨烯 | 亚甲基蓝(MB) | 100 min后去除率为99% | 重复使用8次,稳定性良好 | 在pH为2.0~10.2范围内去除效果良好 | 〔28〕 |
| Fe3O4 | 石墨烯 | 苯酚、2-NP、2-CP | 120 min后苯酚去除率为92.43%,2-NP去除率为98.87%,2-CP去除率为97.15% | 重复使用10次,稳定性良好 | 在pH为3.0~11.0范围内去除效果良好 | 〔29〕 |
| Fe3O4 | 生物炭 | 乙苯 | 40 min后去除率为99.33% | — | 在pH为3~10范围内去除效果良好 | 〔30〕 |
| 纳米零价铁(NZVI) | 树胶黄花胶(GT) | 阿西莫拉(CIP) | 60 min后去除率为85% | — | 在pH为3.5~9.5范围内去除效果良好 | 〔31〕 |
| FeOOH | 活性炭 | 活性艳橙(X-GN) | 240 min后去除率达到98% | 重复使用4次,稳定性良好 | 在pH为2~9范围内去除效果良好 | 〔32〕 |
由表 1可知,铁基催化剂中除了矿物催化剂外,合成纳米零价铁也能作为Fenton反应的催化剂.将催化剂负载在支撑材料上后,其能表现出较高的催化活性,短时间内目标污染物去除率都能达到90%以上,且多数负载铁催化剂的稳定性良好,重复使用5~10次活性没有明显下降.更重要的是,与传统催化剂相比,其适用的pH范围有了极大拓展,从酸性到弱碱性条件下均可以保持原有活性.负载的铁基催化剂通常以Fe2O3、Fe3O4和NZVI为主,其负载到负载材料上后能有效减少铁的浸出和聚集. ...
A novel α-Fe2O3@g-C3N4 catalyst: Synthesis derived from Fe-based MOF and its superior photo-Fenton performance
1
2019
... 常见的负载铁基催化剂在Fenton反应中的应用效果
| 催化剂 | 催化剂支撑材料 | 降解目标物 | 最优结果 | 催化剂稳定性 | 适用pH范围 | 参考文献 |
| α-Fe2O3 | 硅藻土 | 亚甲基蓝(MB) | 120 min后去除率达到98% | 重复使用5次,稳定性良好 | 在pH为2.0~8.0范围内去除效果良好 | 〔26〕 |
| α-Fe2O3 | 石墨相碳氮化物(g-C3N4) | 四环素(TC) | 60 min后去除率为95% | 重复使用5次,稳定性良好 | 在pH为2.01~9.09范围内去除效果良好 | 〔27〕 |
| γ-Fe2O3 | 石墨烯 | 亚甲基蓝(MB) | 100 min后去除率为99% | 重复使用8次,稳定性良好 | 在pH为2.0~10.2范围内去除效果良好 | 〔28〕 |
| Fe3O4 | 石墨烯 | 苯酚、2-NP、2-CP | 120 min后苯酚去除率为92.43%,2-NP去除率为98.87%,2-CP去除率为97.15% | 重复使用10次,稳定性良好 | 在pH为3.0~11.0范围内去除效果良好 | 〔29〕 |
| Fe3O4 | 生物炭 | 乙苯 | 40 min后去除率为99.33% | — | 在pH为3~10范围内去除效果良好 | 〔30〕 |
| 纳米零价铁(NZVI) | 树胶黄花胶(GT) | 阿西莫拉(CIP) | 60 min后去除率为85% | — | 在pH为3.5~9.5范围内去除效果良好 | 〔31〕 |
| FeOOH | 活性炭 | 活性艳橙(X-GN) | 240 min后去除率达到98% | 重复使用4次,稳定性良好 | 在pH为2~9范围内去除效果良好 | 〔32〕 |
由表 1可知,铁基催化剂中除了矿物催化剂外,合成纳米零价铁也能作为Fenton反应的催化剂.将催化剂负载在支撑材料上后,其能表现出较高的催化活性,短时间内目标污染物去除率都能达到90%以上,且多数负载铁催化剂的稳定性良好,重复使用5~10次活性没有明显下降.更重要的是,与传统催化剂相比,其适用的pH范围有了极大拓展,从酸性到弱碱性条件下均可以保持原有活性.负载的铁基催化剂通常以Fe2O3、Fe3O4和NZVI为主,其负载到负载材料上后能有效减少铁的浸出和聚集. ...
Facile self-assembly synthesis of γ-Fe2O3/graphene oxide for enhanced photo-Fenton reaction
1
2019
... 常见的负载铁基催化剂在Fenton反应中的应用效果
| 催化剂 | 催化剂支撑材料 | 降解目标物 | 最优结果 | 催化剂稳定性 | 适用pH范围 | 参考文献 |
| α-Fe2O3 | 硅藻土 | 亚甲基蓝(MB) | 120 min后去除率达到98% | 重复使用5次,稳定性良好 | 在pH为2.0~8.0范围内去除效果良好 | 〔26〕 |
| α-Fe2O3 | 石墨相碳氮化物(g-C3N4) | 四环素(TC) | 60 min后去除率为95% | 重复使用5次,稳定性良好 | 在pH为2.01~9.09范围内去除效果良好 | 〔27〕 |
| γ-Fe2O3 | 石墨烯 | 亚甲基蓝(MB) | 100 min后去除率为99% | 重复使用8次,稳定性良好 | 在pH为2.0~10.2范围内去除效果良好 | 〔28〕 |
| Fe3O4 | 石墨烯 | 苯酚、2-NP、2-CP | 120 min后苯酚去除率为92.43%,2-NP去除率为98.87%,2-CP去除率为97.15% | 重复使用10次,稳定性良好 | 在pH为3.0~11.0范围内去除效果良好 | 〔29〕 |
| Fe3O4 | 生物炭 | 乙苯 | 40 min后去除率为99.33% | — | 在pH为3~10范围内去除效果良好 | 〔30〕 |
| 纳米零价铁(NZVI) | 树胶黄花胶(GT) | 阿西莫拉(CIP) | 60 min后去除率为85% | — | 在pH为3.5~9.5范围内去除效果良好 | 〔31〕 |
| FeOOH | 活性炭 | 活性艳橙(X-GN) | 240 min后去除率达到98% | 重复使用4次,稳定性良好 | 在pH为2~9范围内去除效果良好 | 〔32〕 |
由表 1可知,铁基催化剂中除了矿物催化剂外,合成纳米零价铁也能作为Fenton反应的催化剂.将催化剂负载在支撑材料上后,其能表现出较高的催化活性,短时间内目标污染物去除率都能达到90%以上,且多数负载铁催化剂的稳定性良好,重复使用5~10次活性没有明显下降.更重要的是,与传统催化剂相比,其适用的pH范围有了极大拓展,从酸性到弱碱性条件下均可以保持原有活性.负载的铁基催化剂通常以Fe2O3、Fe3O4和NZVI为主,其负载到负载材料上后能有效减少铁的浸出和聚集. ...
Ammonia-modified graphene sheets decorated with magnetic Fe3O4 nanoparticles for the photocatalytic and photo-Fenton degradation of phenolic compounds under sunlight irradiation
1
2017
... 常见的负载铁基催化剂在Fenton反应中的应用效果
| 催化剂 | 催化剂支撑材料 | 降解目标物 | 最优结果 | 催化剂稳定性 | 适用pH范围 | 参考文献 |
| α-Fe2O3 | 硅藻土 | 亚甲基蓝(MB) | 120 min后去除率达到98% | 重复使用5次,稳定性良好 | 在pH为2.0~8.0范围内去除效果良好 | 〔26〕 |
| α-Fe2O3 | 石墨相碳氮化物(g-C3N4) | 四环素(TC) | 60 min后去除率为95% | 重复使用5次,稳定性良好 | 在pH为2.01~9.09范围内去除效果良好 | 〔27〕 |
| γ-Fe2O3 | 石墨烯 | 亚甲基蓝(MB) | 100 min后去除率为99% | 重复使用8次,稳定性良好 | 在pH为2.0~10.2范围内去除效果良好 | 〔28〕 |
| Fe3O4 | 石墨烯 | 苯酚、2-NP、2-CP | 120 min后苯酚去除率为92.43%,2-NP去除率为98.87%,2-CP去除率为97.15% | 重复使用10次,稳定性良好 | 在pH为3.0~11.0范围内去除效果良好 | 〔29〕 |
| Fe3O4 | 生物炭 | 乙苯 | 40 min后去除率为99.33% | — | 在pH为3~10范围内去除效果良好 | 〔30〕 |
| 纳米零价铁(NZVI) | 树胶黄花胶(GT) | 阿西莫拉(CIP) | 60 min后去除率为85% | — | 在pH为3.5~9.5范围内去除效果良好 | 〔31〕 |
| FeOOH | 活性炭 | 活性艳橙(X-GN) | 240 min后去除率达到98% | 重复使用4次,稳定性良好 | 在pH为2~9范围内去除效果良好 | 〔32〕 |
由表 1可知,铁基催化剂中除了矿物催化剂外,合成纳米零价铁也能作为Fenton反应的催化剂.将催化剂负载在支撑材料上后,其能表现出较高的催化活性,短时间内目标污染物去除率都能达到90%以上,且多数负载铁催化剂的稳定性良好,重复使用5~10次活性没有明显下降.更重要的是,与传统催化剂相比,其适用的pH范围有了极大拓展,从酸性到弱碱性条件下均可以保持原有活性.负载的铁基催化剂通常以Fe2O3、Fe3O4和NZVI为主,其负载到负载材料上后能有效减少铁的浸出和聚集. ...
Nano-magnetite supported by biochar pyrolyzed at different temperatures as hydrogen peroxide activator: Synthesis mechanism and the effects on ethylbenzene removal
1
2020
... 常见的负载铁基催化剂在Fenton反应中的应用效果
| 催化剂 | 催化剂支撑材料 | 降解目标物 | 最优结果 | 催化剂稳定性 | 适用pH范围 | 参考文献 |
| α-Fe2O3 | 硅藻土 | 亚甲基蓝(MB) | 120 min后去除率达到98% | 重复使用5次,稳定性良好 | 在pH为2.0~8.0范围内去除效果良好 | 〔26〕 |
| α-Fe2O3 | 石墨相碳氮化物(g-C3N4) | 四环素(TC) | 60 min后去除率为95% | 重复使用5次,稳定性良好 | 在pH为2.01~9.09范围内去除效果良好 | 〔27〕 |
| γ-Fe2O3 | 石墨烯 | 亚甲基蓝(MB) | 100 min后去除率为99% | 重复使用8次,稳定性良好 | 在pH为2.0~10.2范围内去除效果良好 | 〔28〕 |
| Fe3O4 | 石墨烯 | 苯酚、2-NP、2-CP | 120 min后苯酚去除率为92.43%,2-NP去除率为98.87%,2-CP去除率为97.15% | 重复使用10次,稳定性良好 | 在pH为3.0~11.0范围内去除效果良好 | 〔29〕 |
| Fe3O4 | 生物炭 | 乙苯 | 40 min后去除率为99.33% | — | 在pH为3~10范围内去除效果良好 | 〔30〕 |
| 纳米零价铁(NZVI) | 树胶黄花胶(GT) | 阿西莫拉(CIP) | 60 min后去除率为85% | — | 在pH为3.5~9.5范围内去除效果良好 | 〔31〕 |
| FeOOH | 活性炭 | 活性艳橙(X-GN) | 240 min后去除率达到98% | 重复使用4次,稳定性良好 | 在pH为2~9范围内去除效果良好 | 〔32〕 |
由表 1可知,铁基催化剂中除了矿物催化剂外,合成纳米零价铁也能作为Fenton反应的催化剂.将催化剂负载在支撑材料上后,其能表现出较高的催化活性,短时间内目标污染物去除率都能达到90%以上,且多数负载铁催化剂的稳定性良好,重复使用5~10次活性没有明显下降.更重要的是,与传统催化剂相比,其适用的pH范围有了极大拓展,从酸性到弱碱性条件下均可以保持原有活性.负载的铁基催化剂通常以Fe2O3、Fe3O4和NZVI为主,其负载到负载材料上后能有效减少铁的浸出和聚集. ...
A new composite of nano zero-valent iron encapsulated in carbon dots for oxidative removal of bio-refractory antibiotics from water
1
2019
... 常见的负载铁基催化剂在Fenton反应中的应用效果
| 催化剂 | 催化剂支撑材料 | 降解目标物 | 最优结果 | 催化剂稳定性 | 适用pH范围 | 参考文献 |
| α-Fe2O3 | 硅藻土 | 亚甲基蓝(MB) | 120 min后去除率达到98% | 重复使用5次,稳定性良好 | 在pH为2.0~8.0范围内去除效果良好 | 〔26〕 |
| α-Fe2O3 | 石墨相碳氮化物(g-C3N4) | 四环素(TC) | 60 min后去除率为95% | 重复使用5次,稳定性良好 | 在pH为2.01~9.09范围内去除效果良好 | 〔27〕 |
| γ-Fe2O3 | 石墨烯 | 亚甲基蓝(MB) | 100 min后去除率为99% | 重复使用8次,稳定性良好 | 在pH为2.0~10.2范围内去除效果良好 | 〔28〕 |
| Fe3O4 | 石墨烯 | 苯酚、2-NP、2-CP | 120 min后苯酚去除率为92.43%,2-NP去除率为98.87%,2-CP去除率为97.15% | 重复使用10次,稳定性良好 | 在pH为3.0~11.0范围内去除效果良好 | 〔29〕 |
| Fe3O4 | 生物炭 | 乙苯 | 40 min后去除率为99.33% | — | 在pH为3~10范围内去除效果良好 | 〔30〕 |
| 纳米零价铁(NZVI) | 树胶黄花胶(GT) | 阿西莫拉(CIP) | 60 min后去除率为85% | — | 在pH为3.5~9.5范围内去除效果良好 | 〔31〕 |
| FeOOH | 活性炭 | 活性艳橙(X-GN) | 240 min后去除率达到98% | 重复使用4次,稳定性良好 | 在pH为2~9范围内去除效果良好 | 〔32〕 |
由表 1可知,铁基催化剂中除了矿物催化剂外,合成纳米零价铁也能作为Fenton反应的催化剂.将催化剂负载在支撑材料上后,其能表现出较高的催化活性,短时间内目标污染物去除率都能达到90%以上,且多数负载铁催化剂的稳定性良好,重复使用5~10次活性没有明显下降.更重要的是,与传统催化剂相比,其适用的pH范围有了极大拓展,从酸性到弱碱性条件下均可以保持原有活性.负载的铁基催化剂通常以Fe2O3、Fe3O4和NZVI为主,其负载到负载材料上后能有效减少铁的浸出和聚集. ...
Heterogeneous Fenton-like degradation of an azo dye reactive brilliant orange by the combination of activated carbon-FeOOH catalyst and H2O2
1
2013
... 常见的负载铁基催化剂在Fenton反应中的应用效果
| 催化剂 | 催化剂支撑材料 | 降解目标物 | 最优结果 | 催化剂稳定性 | 适用pH范围 | 参考文献 |
| α-Fe2O3 | 硅藻土 | 亚甲基蓝(MB) | 120 min后去除率达到98% | 重复使用5次,稳定性良好 | 在pH为2.0~8.0范围内去除效果良好 | 〔26〕 |
| α-Fe2O3 | 石墨相碳氮化物(g-C3N4) | 四环素(TC) | 60 min后去除率为95% | 重复使用5次,稳定性良好 | 在pH为2.01~9.09范围内去除效果良好 | 〔27〕 |
| γ-Fe2O3 | 石墨烯 | 亚甲基蓝(MB) | 100 min后去除率为99% | 重复使用8次,稳定性良好 | 在pH为2.0~10.2范围内去除效果良好 | 〔28〕 |
| Fe3O4 | 石墨烯 | 苯酚、2-NP、2-CP | 120 min后苯酚去除率为92.43%,2-NP去除率为98.87%,2-CP去除率为97.15% | 重复使用10次,稳定性良好 | 在pH为3.0~11.0范围内去除效果良好 | 〔29〕 |
| Fe3O4 | 生物炭 | 乙苯 | 40 min后去除率为99.33% | — | 在pH为3~10范围内去除效果良好 | 〔30〕 |
| 纳米零价铁(NZVI) | 树胶黄花胶(GT) | 阿西莫拉(CIP) | 60 min后去除率为85% | — | 在pH为3.5~9.5范围内去除效果良好 | 〔31〕 |
| FeOOH | 活性炭 | 活性艳橙(X-GN) | 240 min后去除率达到98% | 重复使用4次,稳定性良好 | 在pH为2~9范围内去除效果良好 | 〔32〕 |
由表 1可知,铁基催化剂中除了矿物催化剂外,合成纳米零价铁也能作为Fenton反应的催化剂.将催化剂负载在支撑材料上后,其能表现出较高的催化活性,短时间内目标污染物去除率都能达到90%以上,且多数负载铁催化剂的稳定性良好,重复使用5~10次活性没有明显下降.更重要的是,与传统催化剂相比,其适用的pH范围有了极大拓展,从酸性到弱碱性条件下均可以保持原有活性.负载的铁基催化剂通常以Fe2O3、Fe3O4和NZVI为主,其负载到负载材料上后能有效减少铁的浸出和聚集. ...
Fenton-like degradation of Bisphenol A catalyzed by mesoporous Cu/TUD-1
1
2017
... 具有多个氧化还原态的金属元素可以替代铁基催化剂参与Fenton反应,它们在较高的pH范围内也能有效降解有机污染物.这些金属能象铁一样作为催化剂催化H2O2产生具有高氧化性的自由基.类似Fenton反应中的铁基催化剂,M. P. Pachamuthu等〔33〕将氧化铜催化剂分散在三维介孔TUD-1二氧化硅上,制得Cu / TUD-1.使用该材料作为Fenton反应催化剂降解水中双酚A(BPA),结果表明,在一定的试验条件下,在180 min内BPA去除率达到90.4%;采用DMPO(5,5-二甲基-1-吡咯啉-N-氧化物)作为自由基猝灭剂进行了额外的电子顺磁共振(EPR)实验,确定了羟基自由基的形成.Peng Zhou等〔34〕使用纳米级零价钨(NZVW)作为类Fenton催化剂,成功去除了溶液中的罗丹明B(RhB),并且在溶液中同样测出了羟基自由基.Chengjie Zang等〔35〕使用二氧化铈作为催化剂,建立了CeO2-H2O2类Fenton体系.该体系可有效降解溶液中的水杨酸(SA),在一定试验条件下,当pH=4.0时,SA降解率达到80%,pH=7.0时,也有50%的SA降解率.有些金属催化剂甚至能表现出比铁基催化剂更好的性能.Yunjin Yao等〔36〕分别将铁、钴、镍包裹在掺氮碳纳米管(NC)中,制得新型Fenton催化剂,并以酸性橙Ⅱ为处理对象,比较了各金属催化剂的催化活性.结果表明:相比于其他2种金属催化剂,Co@NC催化剂能提供更多的反应位点,拥有最高活性,对酸性橙Ⅱ的去除效果最好.催化剂的活性不仅与其表面积有关,而且和催化剂的结晶度也有关.E. J. Kim等〔37〕通过水热法制备了4种不同的棒状MnO2多晶型物:α-MnO2、β-MnO2、γ-MnO2(成隧道状)和δ-MnO2(成层),并测试了它们作为Fenton催化剂降解MB的性能.结果表明,γ-MnO2显示出最高的催化活性,在pH为4.5~10.0的范围内,20 min内能完全去除MB. ...
Removal of Rhodamine B during the corrosion of zero valent tungsten via a tungsten speciescatalyzed Fenton-like system
1
2019
... 具有多个氧化还原态的金属元素可以替代铁基催化剂参与Fenton反应,它们在较高的pH范围内也能有效降解有机污染物.这些金属能象铁一样作为催化剂催化H2O2产生具有高氧化性的自由基.类似Fenton反应中的铁基催化剂,M. P. Pachamuthu等〔33〕将氧化铜催化剂分散在三维介孔TUD-1二氧化硅上,制得Cu / TUD-1.使用该材料作为Fenton反应催化剂降解水中双酚A(BPA),结果表明,在一定的试验条件下,在180 min内BPA去除率达到90.4%;采用DMPO(5,5-二甲基-1-吡咯啉-N-氧化物)作为自由基猝灭剂进行了额外的电子顺磁共振(EPR)实验,确定了羟基自由基的形成.Peng Zhou等〔34〕使用纳米级零价钨(NZVW)作为类Fenton催化剂,成功去除了溶液中的罗丹明B(RhB),并且在溶液中同样测出了羟基自由基.Chengjie Zang等〔35〕使用二氧化铈作为催化剂,建立了CeO2-H2O2类Fenton体系.该体系可有效降解溶液中的水杨酸(SA),在一定试验条件下,当pH=4.0时,SA降解率达到80%,pH=7.0时,也有50%的SA降解率.有些金属催化剂甚至能表现出比铁基催化剂更好的性能.Yunjin Yao等〔36〕分别将铁、钴、镍包裹在掺氮碳纳米管(NC)中,制得新型Fenton催化剂,并以酸性橙Ⅱ为处理对象,比较了各金属催化剂的催化活性.结果表明:相比于其他2种金属催化剂,Co@NC催化剂能提供更多的反应位点,拥有最高活性,对酸性橙Ⅱ的去除效果最好.催化剂的活性不仅与其表面积有关,而且和催化剂的结晶度也有关.E. J. Kim等〔37〕通过水热法制备了4种不同的棒状MnO2多晶型物:α-MnO2、β-MnO2、γ-MnO2(成隧道状)和δ-MnO2(成层),并测试了它们作为Fenton催化剂降解MB的性能.结果表明,γ-MnO2显示出最高的催化活性,在pH为4.5~10.0的范围内,20 min内能完全去除MB. ...
Adsorption-depended Fenton-like reaction kinetics in CeO2-H2O2 system for salicylic acid degradation
1
2018
... 具有多个氧化还原态的金属元素可以替代铁基催化剂参与Fenton反应,它们在较高的pH范围内也能有效降解有机污染物.这些金属能象铁一样作为催化剂催化H2O2产生具有高氧化性的自由基.类似Fenton反应中的铁基催化剂,M. P. Pachamuthu等〔33〕将氧化铜催化剂分散在三维介孔TUD-1二氧化硅上,制得Cu / TUD-1.使用该材料作为Fenton反应催化剂降解水中双酚A(BPA),结果表明,在一定的试验条件下,在180 min内BPA去除率达到90.4%;采用DMPO(5,5-二甲基-1-吡咯啉-N-氧化物)作为自由基猝灭剂进行了额外的电子顺磁共振(EPR)实验,确定了羟基自由基的形成.Peng Zhou等〔34〕使用纳米级零价钨(NZVW)作为类Fenton催化剂,成功去除了溶液中的罗丹明B(RhB),并且在溶液中同样测出了羟基自由基.Chengjie Zang等〔35〕使用二氧化铈作为催化剂,建立了CeO2-H2O2类Fenton体系.该体系可有效降解溶液中的水杨酸(SA),在一定试验条件下,当pH=4.0时,SA降解率达到80%,pH=7.0时,也有50%的SA降解率.有些金属催化剂甚至能表现出比铁基催化剂更好的性能.Yunjin Yao等〔36〕分别将铁、钴、镍包裹在掺氮碳纳米管(NC)中,制得新型Fenton催化剂,并以酸性橙Ⅱ为处理对象,比较了各金属催化剂的催化活性.结果表明:相比于其他2种金属催化剂,Co@NC催化剂能提供更多的反应位点,拥有最高活性,对酸性橙Ⅱ的去除效果最好.催化剂的活性不仅与其表面积有关,而且和催化剂的结晶度也有关.E. J. Kim等〔37〕通过水热法制备了4种不同的棒状MnO2多晶型物:α-MnO2、β-MnO2、γ-MnO2(成隧道状)和δ-MnO2(成层),并测试了它们作为Fenton催化剂降解MB的性能.结果表明,γ-MnO2显示出最高的催化活性,在pH为4.5~10.0的范围内,20 min内能完全去除MB. ...
Fe, Co, Ni nanocrystals encapsulated in nitrogen-doped carbon nanotubes as Fenton-like catalysts for organic pollutant removal
1
2016
... 具有多个氧化还原态的金属元素可以替代铁基催化剂参与Fenton反应,它们在较高的pH范围内也能有效降解有机污染物.这些金属能象铁一样作为催化剂催化H2O2产生具有高氧化性的自由基.类似Fenton反应中的铁基催化剂,M. P. Pachamuthu等〔33〕将氧化铜催化剂分散在三维介孔TUD-1二氧化硅上,制得Cu / TUD-1.使用该材料作为Fenton反应催化剂降解水中双酚A(BPA),结果表明,在一定的试验条件下,在180 min内BPA去除率达到90.4%;采用DMPO(5,5-二甲基-1-吡咯啉-N-氧化物)作为自由基猝灭剂进行了额外的电子顺磁共振(EPR)实验,确定了羟基自由基的形成.Peng Zhou等〔34〕使用纳米级零价钨(NZVW)作为类Fenton催化剂,成功去除了溶液中的罗丹明B(RhB),并且在溶液中同样测出了羟基自由基.Chengjie Zang等〔35〕使用二氧化铈作为催化剂,建立了CeO2-H2O2类Fenton体系.该体系可有效降解溶液中的水杨酸(SA),在一定试验条件下,当pH=4.0时,SA降解率达到80%,pH=7.0时,也有50%的SA降解率.有些金属催化剂甚至能表现出比铁基催化剂更好的性能.Yunjin Yao等〔36〕分别将铁、钴、镍包裹在掺氮碳纳米管(NC)中,制得新型Fenton催化剂,并以酸性橙Ⅱ为处理对象,比较了各金属催化剂的催化活性.结果表明:相比于其他2种金属催化剂,Co@NC催化剂能提供更多的反应位点,拥有最高活性,对酸性橙Ⅱ的去除效果最好.催化剂的活性不仅与其表面积有关,而且和催化剂的结晶度也有关.E. J. Kim等〔37〕通过水热法制备了4种不同的棒状MnO2多晶型物:α-MnO2、β-MnO2、γ-MnO2(成隧道状)和δ-MnO2(成层),并测试了它们作为Fenton催化剂降解MB的性能.结果表明,γ-MnO2显示出最高的催化活性,在pH为4.5~10.0的范围内,20 min内能完全去除MB. ...
Manganese oxide nanorods as a robust Fenton-like catalyst at neutral pH: Crystal phase-dependent behavior
2
2017
... 具有多个氧化还原态的金属元素可以替代铁基催化剂参与Fenton反应,它们在较高的pH范围内也能有效降解有机污染物.这些金属能象铁一样作为催化剂催化H2O2产生具有高氧化性的自由基.类似Fenton反应中的铁基催化剂,M. P. Pachamuthu等〔33〕将氧化铜催化剂分散在三维介孔TUD-1二氧化硅上,制得Cu / TUD-1.使用该材料作为Fenton反应催化剂降解水中双酚A(BPA),结果表明,在一定的试验条件下,在180 min内BPA去除率达到90.4%;采用DMPO(5,5-二甲基-1-吡咯啉-N-氧化物)作为自由基猝灭剂进行了额外的电子顺磁共振(EPR)实验,确定了羟基自由基的形成.Peng Zhou等〔34〕使用纳米级零价钨(NZVW)作为类Fenton催化剂,成功去除了溶液中的罗丹明B(RhB),并且在溶液中同样测出了羟基自由基.Chengjie Zang等〔35〕使用二氧化铈作为催化剂,建立了CeO2-H2O2类Fenton体系.该体系可有效降解溶液中的水杨酸(SA),在一定试验条件下,当pH=4.0时,SA降解率达到80%,pH=7.0时,也有50%的SA降解率.有些金属催化剂甚至能表现出比铁基催化剂更好的性能.Yunjin Yao等〔36〕分别将铁、钴、镍包裹在掺氮碳纳米管(NC)中,制得新型Fenton催化剂,并以酸性橙Ⅱ为处理对象,比较了各金属催化剂的催化活性.结果表明:相比于其他2种金属催化剂,Co@NC催化剂能提供更多的反应位点,拥有最高活性,对酸性橙Ⅱ的去除效果最好.催化剂的活性不仅与其表面积有关,而且和催化剂的结晶度也有关.E. J. Kim等〔37〕通过水热法制备了4种不同的棒状MnO2多晶型物:α-MnO2、β-MnO2、γ-MnO2(成隧道状)和δ-MnO2(成层),并测试了它们作为Fenton催化剂降解MB的性能.结果表明,γ-MnO2显示出最高的催化活性,在pH为4.5~10.0的范围内,20 min内能完全去除MB. ...
... 非铁基金属催化剂的应用是Fenton体系的一个崭新方向,其有效地补充了Fenton体系在高pH环境下的的应用范围.部分非铁基金属催化剂能在较宽泛的pH范围内进行Fenton反应,且催化活性高于铁基金属催化剂,这可能与催化剂的表面积、结晶度、晶面等结构因素有关〔37〕.但非铁基金属催化剂也存在一定缺陷:在pH < 4.0的酸性环境中,非铁基金属催化剂活性不高;部分金属毒性较高,在反应中浸出到环境后会带来巨大隐患;成本也远远大于铁基金属催化剂,不宜大量使用. ...
Efficient degradation of sulfamethazine in simulated and real wastewater at slightly basic pH values using Co-SAM-SCS/H2O2 Fenton-like system
2
2018
... 单金属催化剂所主导的Fenton体系已不能满足日益复杂的污染环境,将2种或2种以上的金属构成的多金属催化剂应用于Fenton体系受到广泛关注.常见的能构筑多金属氧化物的金属有钴〔38〕、锰〔39〕、铈〔40〕、铜〔41〕等.这些金属作为催化剂促进Fenton反应的原理有所不同.如:氧化锰中掺杂铈能显著改善催化剂的表面性能,扩大比表面积,赋予更多的反应位点〔42〕;钒掺杂钙钛矿催化剂能促进具有电子富集特性的氧空位的形成,从而加速电子传输过程和·OH的生成〔43〕.双金属催化剂优于单金属催化剂的主要原因在于双金属催化剂中各金属之间的协同作用有利于·OH的生成.Min Cheng等〔38〕合成了一种新型的Fe/Co复合材料,将其作为类Fenton反应的催化剂催化降解磺胺二甲嘧啶(SMZ).结果表明,在pH为5~9的条件下,Fe/Co复合材料能够加快Fenton反应,对SMZ的降解非常有效.钴离子能与铁离子产生协同作用,加快铁离子之间的循环,使得·OH能更高效生成.X. Zhang等〔44〕用水热法制备了CuFeO2微粒,并将其作为Fenton反应的催化剂催化降解双酚A.结果表明,在一定试验条件下,在120 min内双酚A几乎能得到完全去除.通过使用X射线光电子能谱(XPS)检测Fenton反应前后催化剂上铁和铜的化学状态,证明了催化过程中铁和铜之间的相互作用〔见式(3)〕导致了Fe3+的快速还原. ...
... 〔38〕合成了一种新型的Fe/Co复合材料,将其作为类Fenton反应的催化剂催化降解磺胺二甲嘧啶(SMZ).结果表明,在pH为5~9的条件下,Fe/Co复合材料能够加快Fenton反应,对SMZ的降解非常有效.钴离子能与铁离子产生协同作用,加快铁离子之间的循环,使得·OH能更高效生成.X. Zhang等〔44〕用水热法制备了CuFeO2微粒,并将其作为Fenton反应的催化剂催化降解双酚A.结果表明,在一定试验条件下,在120 min内双酚A几乎能得到完全去除.通过使用X射线光电子能谱(XPS)检测Fenton反应前后催化剂上铁和铜的化学状态,证明了催化过程中铁和铜之间的相互作用〔见式(3)〕导致了Fe3+的快速还原. ...
Degradation of benzotriazole by a novel Fenton-like reaction with mesoporous Cu/MnO2: Combination of adsorption and catalysis oxidation
1
2016
... 单金属催化剂所主导的Fenton体系已不能满足日益复杂的污染环境,将2种或2种以上的金属构成的多金属催化剂应用于Fenton体系受到广泛关注.常见的能构筑多金属氧化物的金属有钴〔38〕、锰〔39〕、铈〔40〕、铜〔41〕等.这些金属作为催化剂促进Fenton反应的原理有所不同.如:氧化锰中掺杂铈能显著改善催化剂的表面性能,扩大比表面积,赋予更多的反应位点〔42〕;钒掺杂钙钛矿催化剂能促进具有电子富集特性的氧空位的形成,从而加速电子传输过程和·OH的生成〔43〕.双金属催化剂优于单金属催化剂的主要原因在于双金属催化剂中各金属之间的协同作用有利于·OH的生成.Min Cheng等〔38〕合成了一种新型的Fe/Co复合材料,将其作为类Fenton反应的催化剂催化降解磺胺二甲嘧啶(SMZ).结果表明,在pH为5~9的条件下,Fe/Co复合材料能够加快Fenton反应,对SMZ的降解非常有效.钴离子能与铁离子产生协同作用,加快铁离子之间的循环,使得·OH能更高效生成.X. Zhang等〔44〕用水热法制备了CuFeO2微粒,并将其作为Fenton反应的催化剂催化降解双酚A.结果表明,在一定试验条件下,在120 min内双酚A几乎能得到完全去除.通过使用X射线光电子能谱(XPS)检测Fenton反应前后催化剂上铁和铜的化学状态,证明了催化过程中铁和铜之间的相互作用〔见式(3)〕导致了Fe3+的快速还原. ...
A novel magnetic nanoscaled Fe3O4/CeO2 composite prepared by oxidation-precipitation process and its application for degradation of orange G in aqueous solution as Fenton-like heterogeneous catalyst
1
2017
... 单金属催化剂所主导的Fenton体系已不能满足日益复杂的污染环境,将2种或2种以上的金属构成的多金属催化剂应用于Fenton体系受到广泛关注.常见的能构筑多金属氧化物的金属有钴〔38〕、锰〔39〕、铈〔40〕、铜〔41〕等.这些金属作为催化剂促进Fenton反应的原理有所不同.如:氧化锰中掺杂铈能显著改善催化剂的表面性能,扩大比表面积,赋予更多的反应位点〔42〕;钒掺杂钙钛矿催化剂能促进具有电子富集特性的氧空位的形成,从而加速电子传输过程和·OH的生成〔43〕.双金属催化剂优于单金属催化剂的主要原因在于双金属催化剂中各金属之间的协同作用有利于·OH的生成.Min Cheng等〔38〕合成了一种新型的Fe/Co复合材料,将其作为类Fenton反应的催化剂催化降解磺胺二甲嘧啶(SMZ).结果表明,在pH为5~9的条件下,Fe/Co复合材料能够加快Fenton反应,对SMZ的降解非常有效.钴离子能与铁离子产生协同作用,加快铁离子之间的循环,使得·OH能更高效生成.X. Zhang等〔44〕用水热法制备了CuFeO2微粒,并将其作为Fenton反应的催化剂催化降解双酚A.结果表明,在一定试验条件下,在120 min内双酚A几乎能得到完全去除.通过使用X射线光电子能谱(XPS)检测Fenton反应前后催化剂上铁和铜的化学状态,证明了催化过程中铁和铜之间的相互作用〔见式(3)〕导致了Fe3+的快速还原. ...
Specifically enhancement of heterogeneous Fenton-like degradation activities for ofloxacin with synergetic effects of bimetallic Fe-Cu on ordered mesoporous silicon
1
2017
... 单金属催化剂所主导的Fenton体系已不能满足日益复杂的污染环境,将2种或2种以上的金属构成的多金属催化剂应用于Fenton体系受到广泛关注.常见的能构筑多金属氧化物的金属有钴〔38〕、锰〔39〕、铈〔40〕、铜〔41〕等.这些金属作为催化剂促进Fenton反应的原理有所不同.如:氧化锰中掺杂铈能显著改善催化剂的表面性能,扩大比表面积,赋予更多的反应位点〔42〕;钒掺杂钙钛矿催化剂能促进具有电子富集特性的氧空位的形成,从而加速电子传输过程和·OH的生成〔43〕.双金属催化剂优于单金属催化剂的主要原因在于双金属催化剂中各金属之间的协同作用有利于·OH的生成.Min Cheng等〔38〕合成了一种新型的Fe/Co复合材料,将其作为类Fenton反应的催化剂催化降解磺胺二甲嘧啶(SMZ).结果表明,在pH为5~9的条件下,Fe/Co复合材料能够加快Fenton反应,对SMZ的降解非常有效.钴离子能与铁离子产生协同作用,加快铁离子之间的循环,使得·OH能更高效生成.X. Zhang等〔44〕用水热法制备了CuFeO2微粒,并将其作为Fenton反应的催化剂催化降解双酚A.结果表明,在一定试验条件下,在120 min内双酚A几乎能得到完全去除.通过使用X射线光电子能谱(XPS)检测Fenton反应前后催化剂上铁和铜的化学状态,证明了催化过程中铁和铜之间的相互作用〔见式(3)〕导致了Fe3+的快速还原. ...
Enhanced catalytic degradation of ciprofloxacin over Ce-doped OMS-2 microspheres
1
2016
... 单金属催化剂所主导的Fenton体系已不能满足日益复杂的污染环境,将2种或2种以上的金属构成的多金属催化剂应用于Fenton体系受到广泛关注.常见的能构筑多金属氧化物的金属有钴〔38〕、锰〔39〕、铈〔40〕、铜〔41〕等.这些金属作为催化剂促进Fenton反应的原理有所不同.如:氧化锰中掺杂铈能显著改善催化剂的表面性能,扩大比表面积,赋予更多的反应位点〔42〕;钒掺杂钙钛矿催化剂能促进具有电子富集特性的氧空位的形成,从而加速电子传输过程和·OH的生成〔43〕.双金属催化剂优于单金属催化剂的主要原因在于双金属催化剂中各金属之间的协同作用有利于·OH的生成.Min Cheng等〔38〕合成了一种新型的Fe/Co复合材料,将其作为类Fenton反应的催化剂催化降解磺胺二甲嘧啶(SMZ).结果表明,在pH为5~9的条件下,Fe/Co复合材料能够加快Fenton反应,对SMZ的降解非常有效.钴离子能与铁离子产生协同作用,加快铁离子之间的循环,使得·OH能更高效生成.X. Zhang等〔44〕用水热法制备了CuFeO2微粒,并将其作为Fenton反应的催化剂催化降解双酚A.结果表明,在一定试验条件下,在120 min内双酚A几乎能得到完全去除.通过使用X射线光电子能谱(XPS)检测Fenton反应前后催化剂上铁和铜的化学状态,证明了催化过程中铁和铜之间的相互作用〔见式(3)〕导致了Fe3+的快速还原. ...
Enhanced degradation of organic pollutants over Cu-doped LaAlO3 perovskite through heterogeneous Fenton-like reactions
1
2018
... 单金属催化剂所主导的Fenton体系已不能满足日益复杂的污染环境,将2种或2种以上的金属构成的多金属催化剂应用于Fenton体系受到广泛关注.常见的能构筑多金属氧化物的金属有钴〔38〕、锰〔39〕、铈〔40〕、铜〔41〕等.这些金属作为催化剂促进Fenton反应的原理有所不同.如:氧化锰中掺杂铈能显著改善催化剂的表面性能,扩大比表面积,赋予更多的反应位点〔42〕;钒掺杂钙钛矿催化剂能促进具有电子富集特性的氧空位的形成,从而加速电子传输过程和·OH的生成〔43〕.双金属催化剂优于单金属催化剂的主要原因在于双金属催化剂中各金属之间的协同作用有利于·OH的生成.Min Cheng等〔38〕合成了一种新型的Fe/Co复合材料,将其作为类Fenton反应的催化剂催化降解磺胺二甲嘧啶(SMZ).结果表明,在pH为5~9的条件下,Fe/Co复合材料能够加快Fenton反应,对SMZ的降解非常有效.钴离子能与铁离子产生协同作用,加快铁离子之间的循环,使得·OH能更高效生成.X. Zhang等〔44〕用水热法制备了CuFeO2微粒,并将其作为Fenton反应的催化剂催化降解双酚A.结果表明,在一定试验条件下,在120 min内双酚A几乎能得到完全去除.通过使用X射线光电子能谱(XPS)检测Fenton反应前后催化剂上铁和铜的化学状态,证明了催化过程中铁和铜之间的相互作用〔见式(3)〕导致了Fe3+的快速还原. ...
Degradation of bisphenol A by hydrogen peroxide activated with CuFeO2 microparticles as a heterogeneous Fenton-like catalyst: Efficiency, stability and mechanism
2
2014
... 单金属催化剂所主导的Fenton体系已不能满足日益复杂的污染环境,将2种或2种以上的金属构成的多金属催化剂应用于Fenton体系受到广泛关注.常见的能构筑多金属氧化物的金属有钴〔38〕、锰〔39〕、铈〔40〕、铜〔41〕等.这些金属作为催化剂促进Fenton反应的原理有所不同.如:氧化锰中掺杂铈能显著改善催化剂的表面性能,扩大比表面积,赋予更多的反应位点〔42〕;钒掺杂钙钛矿催化剂能促进具有电子富集特性的氧空位的形成,从而加速电子传输过程和·OH的生成〔43〕.双金属催化剂优于单金属催化剂的主要原因在于双金属催化剂中各金属之间的协同作用有利于·OH的生成.Min Cheng等〔38〕合成了一种新型的Fe/Co复合材料,将其作为类Fenton反应的催化剂催化降解磺胺二甲嘧啶(SMZ).结果表明,在pH为5~9的条件下,Fe/Co复合材料能够加快Fenton反应,对SMZ的降解非常有效.钴离子能与铁离子产生协同作用,加快铁离子之间的循环,使得·OH能更高效生成.X. Zhang等〔44〕用水热法制备了CuFeO2微粒,并将其作为Fenton反应的催化剂催化降解双酚A.结果表明,在一定试验条件下,在120 min内双酚A几乎能得到完全去除.通过使用X射线光电子能谱(XPS)检测Fenton反应前后催化剂上铁和铜的化学状态,证明了催化过程中铁和铜之间的相互作用〔见式(3)〕导致了Fe3+的快速还原. ...
... 常见的双金属催化剂在Fenton反应中的应用效果
| 催化剂 | 降解目标物 | 反应条件 | 结果 | 催化剂稳定性 | 适用pH范围 | 参考文献 |
FeCeOx
复合材料 | 罗丹明B(RhB) | 催化剂1.5 g/L,[H2O2]=80 mmol/L,pH=5.0,温度35 ℃ | 150 min后去除率达到98% | 重复使用3次,去除率下降至70%;可以通过简单的热处理(500 ℃,2 h)使催化剂重新活化 | 在pH为3.0~9.0范围内去除效果良好 | 〔45〕 |
LFO-15Cu
复合材料 | 甲基橙(MO) | 催化剂0.8 g/L,H2O2 0.3 g/L,pH=5.0,温度25 ℃,可见光 | 60 min后去除率达到92.9% | 重复使用4次,去除率下降至88.5% | 在pH为4.0~8.0范围内去除效果良好 | 〔46〕 |
CuFeO2
微粒 | 双酚A(BPA) | 催化剂1 g/L,[H2O2]=20 mmol/L,pH=5.0 | 120 min后去除率为99.7% | 重复使用6次,去除率没有明显变化 | 在pH为4.0~8.0范围内去除效果良好 | 〔44〕 |
CuVOx
复合材料 | 氟康唑(FLC) | 催化剂1 g/L,[H2O2]=50 mmol/L,pH=3.0 | 90 min后去除率为100% | 重复使用5次,去除率下降至81% | 在pH为3.0~9.0范围内去除效果良好 | 〔47〕 |
Fe0.75Cu0.25
(BDC) | 磺胺甲恶唑(SMX) | 催化剂0.5 g/L,[H2O2]=6 mmol/L,pH=5.6,温度25 ℃ | 120 min后去除率为100% | 重复使用3次,去除率没有明显变化 | 在pH为4.0~8.6范围内去除效果良好 | 〔48〕 |
双金属催化剂种类繁多,常见的双金属催化剂可分为2类:(1)铁基双金属催化剂.其以铁为基础掺杂其他金属作为催化剂,掺杂金属可加速Fe(Ⅲ)与Fe(Ⅱ)之间的转换,从而提高反应速率.(2)铜基双金属催化剂.铜离子在Fenton反应中体现出耐盐性〔49〕,并有高溶解度〔50〕特性,使得铜在高pH、高盐条件下表现出比铁更高的活性.铜离子参与的Fenton反应与Fe2+/H2O2和Fe3+/H2O2体系的Fenton反应相似〔见式(4)和式(5)〕,并且Cu(Ⅱ)/Cu(Ⅰ)的标准还原电位低于Fe(Ⅲ)/ Fe(Ⅱ)的还原电位,铜基催化剂更容易促进羟基自由基的产生,在热力学上更有利于Fenton系统降解污染物〔51〕.近年来,为了适应复杂的污染环境,铜基双金属催化剂已经逐渐替代铁基双金属催化剂成为研究的重点. ...
Critical role of oxygen vacancies in heterogeneous Fenton oxidation over ceria-based catalysts
1
2020
... 常见的双金属催化剂在Fenton反应中的应用效果
| 催化剂 | 降解目标物 | 反应条件 | 结果 | 催化剂稳定性 | 适用pH范围 | 参考文献 |
FeCeOx
复合材料 | 罗丹明B(RhB) | 催化剂1.5 g/L,[H2O2]=80 mmol/L,pH=5.0,温度35 ℃ | 150 min后去除率达到98% | 重复使用3次,去除率下降至70%;可以通过简单的热处理(500 ℃,2 h)使催化剂重新活化 | 在pH为3.0~9.0范围内去除效果良好 | 〔45〕 |
LFO-15Cu
复合材料 | 甲基橙(MO) | 催化剂0.8 g/L,H2O2 0.3 g/L,pH=5.0,温度25 ℃,可见光 | 60 min后去除率达到92.9% | 重复使用4次,去除率下降至88.5% | 在pH为4.0~8.0范围内去除效果良好 | 〔46〕 |
CuFeO2
微粒 | 双酚A(BPA) | 催化剂1 g/L,[H2O2]=20 mmol/L,pH=5.0 | 120 min后去除率为99.7% | 重复使用6次,去除率没有明显变化 | 在pH为4.0~8.0范围内去除效果良好 | 〔44〕 |
CuVOx
复合材料 | 氟康唑(FLC) | 催化剂1 g/L,[H2O2]=50 mmol/L,pH=3.0 | 90 min后去除率为100% | 重复使用5次,去除率下降至81% | 在pH为3.0~9.0范围内去除效果良好 | 〔47〕 |
Fe0.75Cu0.25
(BDC) | 磺胺甲恶唑(SMX) | 催化剂0.5 g/L,[H2O2]=6 mmol/L,pH=5.6,温度25 ℃ | 120 min后去除率为100% | 重复使用3次,去除率没有明显变化 | 在pH为4.0~8.6范围内去除效果良好 | 〔48〕 |
双金属催化剂种类繁多,常见的双金属催化剂可分为2类:(1)铁基双金属催化剂.其以铁为基础掺杂其他金属作为催化剂,掺杂金属可加速Fe(Ⅲ)与Fe(Ⅱ)之间的转换,从而提高反应速率.(2)铜基双金属催化剂.铜离子在Fenton反应中体现出耐盐性〔49〕,并有高溶解度〔50〕特性,使得铜在高pH、高盐条件下表现出比铁更高的活性.铜离子参与的Fenton反应与Fe2+/H2O2和Fe3+/H2O2体系的Fenton反应相似〔见式(4)和式(5)〕,并且Cu(Ⅱ)/Cu(Ⅰ)的标准还原电位低于Fe(Ⅲ)/ Fe(Ⅱ)的还原电位,铜基催化剂更容易促进羟基自由基的产生,在热力学上更有利于Fenton系统降解污染物〔51〕.近年来,为了适应复杂的污染环境,铜基双金属催化剂已经逐渐替代铁基双金属催化剂成为研究的重点. ...
Heterogeneous photo-Fenton degradation of organics using highly efficient Cu-doped LaFeO3 under visible light
1
2018
... 常见的双金属催化剂在Fenton反应中的应用效果
| 催化剂 | 降解目标物 | 反应条件 | 结果 | 催化剂稳定性 | 适用pH范围 | 参考文献 |
FeCeOx
复合材料 | 罗丹明B(RhB) | 催化剂1.5 g/L,[H2O2]=80 mmol/L,pH=5.0,温度35 ℃ | 150 min后去除率达到98% | 重复使用3次,去除率下降至70%;可以通过简单的热处理(500 ℃,2 h)使催化剂重新活化 | 在pH为3.0~9.0范围内去除效果良好 | 〔45〕 |
LFO-15Cu
复合材料 | 甲基橙(MO) | 催化剂0.8 g/L,H2O2 0.3 g/L,pH=5.0,温度25 ℃,可见光 | 60 min后去除率达到92.9% | 重复使用4次,去除率下降至88.5% | 在pH为4.0~8.0范围内去除效果良好 | 〔46〕 |
CuFeO2
微粒 | 双酚A(BPA) | 催化剂1 g/L,[H2O2]=20 mmol/L,pH=5.0 | 120 min后去除率为99.7% | 重复使用6次,去除率没有明显变化 | 在pH为4.0~8.0范围内去除效果良好 | 〔44〕 |
CuVOx
复合材料 | 氟康唑(FLC) | 催化剂1 g/L,[H2O2]=50 mmol/L,pH=3.0 | 90 min后去除率为100% | 重复使用5次,去除率下降至81% | 在pH为3.0~9.0范围内去除效果良好 | 〔47〕 |
Fe0.75Cu0.25
(BDC) | 磺胺甲恶唑(SMX) | 催化剂0.5 g/L,[H2O2]=6 mmol/L,pH=5.6,温度25 ℃ | 120 min后去除率为100% | 重复使用3次,去除率没有明显变化 | 在pH为4.0~8.6范围内去除效果良好 | 〔48〕 |
双金属催化剂种类繁多,常见的双金属催化剂可分为2类:(1)铁基双金属催化剂.其以铁为基础掺杂其他金属作为催化剂,掺杂金属可加速Fe(Ⅲ)与Fe(Ⅱ)之间的转换,从而提高反应速率.(2)铜基双金属催化剂.铜离子在Fenton反应中体现出耐盐性〔49〕,并有高溶解度〔50〕特性,使得铜在高pH、高盐条件下表现出比铁更高的活性.铜离子参与的Fenton反应与Fe2+/H2O2和Fe3+/H2O2体系的Fenton反应相似〔见式(4)和式(5)〕,并且Cu(Ⅱ)/Cu(Ⅰ)的标准还原电位低于Fe(Ⅲ)/ Fe(Ⅱ)的还原电位,铜基催化剂更容易促进羟基自由基的产生,在热力学上更有利于Fenton系统降解污染物〔51〕.近年来,为了适应复杂的污染环境,铜基双金属催化剂已经逐渐替代铁基双金属催化剂成为研究的重点. ...
Efficient oxidative degradation of fluconazole by a heterogeneous Fenton process with Cu-V bimetallic catalysts
1
2020
... 常见的双金属催化剂在Fenton反应中的应用效果
| 催化剂 | 降解目标物 | 反应条件 | 结果 | 催化剂稳定性 | 适用pH范围 | 参考文献 |
FeCeOx
复合材料 | 罗丹明B(RhB) | 催化剂1.5 g/L,[H2O2]=80 mmol/L,pH=5.0,温度35 ℃ | 150 min后去除率达到98% | 重复使用3次,去除率下降至70%;可以通过简单的热处理(500 ℃,2 h)使催化剂重新活化 | 在pH为3.0~9.0范围内去除效果良好 | 〔45〕 |
LFO-15Cu
复合材料 | 甲基橙(MO) | 催化剂0.8 g/L,H2O2 0.3 g/L,pH=5.0,温度25 ℃,可见光 | 60 min后去除率达到92.9% | 重复使用4次,去除率下降至88.5% | 在pH为4.0~8.0范围内去除效果良好 | 〔46〕 |
CuFeO2
微粒 | 双酚A(BPA) | 催化剂1 g/L,[H2O2]=20 mmol/L,pH=5.0 | 120 min后去除率为99.7% | 重复使用6次,去除率没有明显变化 | 在pH为4.0~8.0范围内去除效果良好 | 〔44〕 |
CuVOx
复合材料 | 氟康唑(FLC) | 催化剂1 g/L,[H2O2]=50 mmol/L,pH=3.0 | 90 min后去除率为100% | 重复使用5次,去除率下降至81% | 在pH为3.0~9.0范围内去除效果良好 | 〔47〕 |
Fe0.75Cu0.25
(BDC) | 磺胺甲恶唑(SMX) | 催化剂0.5 g/L,[H2O2]=6 mmol/L,pH=5.6,温度25 ℃ | 120 min后去除率为100% | 重复使用3次,去除率没有明显变化 | 在pH为4.0~8.6范围内去除效果良好 | 〔48〕 |
双金属催化剂种类繁多,常见的双金属催化剂可分为2类:(1)铁基双金属催化剂.其以铁为基础掺杂其他金属作为催化剂,掺杂金属可加速Fe(Ⅲ)与Fe(Ⅱ)之间的转换,从而提高反应速率.(2)铜基双金属催化剂.铜离子在Fenton反应中体现出耐盐性〔49〕,并有高溶解度〔50〕特性,使得铜在高pH、高盐条件下表现出比铁更高的活性.铜离子参与的Fenton反应与Fe2+/H2O2和Fe3+/H2O2体系的Fenton反应相似〔见式(4)和式(5)〕,并且Cu(Ⅱ)/Cu(Ⅰ)的标准还原电位低于Fe(Ⅲ)/ Fe(Ⅱ)的还原电位,铜基催化剂更容易促进羟基自由基的产生,在热力学上更有利于Fenton系统降解污染物〔51〕.近年来,为了适应复杂的污染环境,铜基双金属催化剂已经逐渐替代铁基双金属催化剂成为研究的重点. ...
Iron-copper bimetallic metal-organic frameworks for efficient Fenton-like degradation of sulfamethoxazole under mild conditions
1
2020
... 常见的双金属催化剂在Fenton反应中的应用效果
| 催化剂 | 降解目标物 | 反应条件 | 结果 | 催化剂稳定性 | 适用pH范围 | 参考文献 |
FeCeOx
复合材料 | 罗丹明B(RhB) | 催化剂1.5 g/L,[H2O2]=80 mmol/L,pH=5.0,温度35 ℃ | 150 min后去除率达到98% | 重复使用3次,去除率下降至70%;可以通过简单的热处理(500 ℃,2 h)使催化剂重新活化 | 在pH为3.0~9.0范围内去除效果良好 | 〔45〕 |
LFO-15Cu
复合材料 | 甲基橙(MO) | 催化剂0.8 g/L,H2O2 0.3 g/L,pH=5.0,温度25 ℃,可见光 | 60 min后去除率达到92.9% | 重复使用4次,去除率下降至88.5% | 在pH为4.0~8.0范围内去除效果良好 | 〔46〕 |
CuFeO2
微粒 | 双酚A(BPA) | 催化剂1 g/L,[H2O2]=20 mmol/L,pH=5.0 | 120 min后去除率为99.7% | 重复使用6次,去除率没有明显变化 | 在pH为4.0~8.0范围内去除效果良好 | 〔44〕 |
CuVOx
复合材料 | 氟康唑(FLC) | 催化剂1 g/L,[H2O2]=50 mmol/L,pH=3.0 | 90 min后去除率为100% | 重复使用5次,去除率下降至81% | 在pH为3.0~9.0范围内去除效果良好 | 〔47〕 |
Fe0.75Cu0.25
(BDC) | 磺胺甲恶唑(SMX) | 催化剂0.5 g/L,[H2O2]=6 mmol/L,pH=5.6,温度25 ℃ | 120 min后去除率为100% | 重复使用3次,去除率没有明显变化 | 在pH为4.0~8.6范围内去除效果良好 | 〔48〕 |
双金属催化剂种类繁多,常见的双金属催化剂可分为2类:(1)铁基双金属催化剂.其以铁为基础掺杂其他金属作为催化剂,掺杂金属可加速Fe(Ⅲ)与Fe(Ⅱ)之间的转换,从而提高反应速率.(2)铜基双金属催化剂.铜离子在Fenton反应中体现出耐盐性〔49〕,并有高溶解度〔50〕特性,使得铜在高pH、高盐条件下表现出比铁更高的活性.铜离子参与的Fenton反应与Fe2+/H2O2和Fe3+/H2O2体系的Fenton反应相似〔见式(4)和式(5)〕,并且Cu(Ⅱ)/Cu(Ⅰ)的标准还原电位低于Fe(Ⅲ)/ Fe(Ⅱ)的还原电位,铜基催化剂更容易促进羟基自由基的产生,在热力学上更有利于Fenton系统降解污染物〔51〕.近年来,为了适应复杂的污染环境,铜基双金属催化剂已经逐渐替代铁基双金属催化剂成为研究的重点. ...
Accelerated oxidation of 2, 4, 6-trichlorophenol in Cu(Ⅱ)/H2O2/Cl- system: A unique "halotolerant" Fenton-like process?
1
2019
... 双金属催化剂种类繁多,常见的双金属催化剂可分为2类:(1)铁基双金属催化剂.其以铁为基础掺杂其他金属作为催化剂,掺杂金属可加速Fe(Ⅲ)与Fe(Ⅱ)之间的转换,从而提高反应速率.(2)铜基双金属催化剂.铜离子在Fenton反应中体现出耐盐性〔49〕,并有高溶解度〔50〕特性,使得铜在高pH、高盐条件下表现出比铁更高的活性.铜离子参与的Fenton反应与Fe2+/H2O2和Fe3+/H2O2体系的Fenton反应相似〔见式(4)和式(5)〕,并且Cu(Ⅱ)/Cu(Ⅰ)的标准还原电位低于Fe(Ⅲ)/ Fe(Ⅱ)的还原电位,铜基催化剂更容易促进羟基自由基的产生,在热力学上更有利于Fenton系统降解污染物〔51〕.近年来,为了适应复杂的污染环境,铜基双金属催化剂已经逐渐替代铁基双金属催化剂成为研究的重点. ...
pH-Dependent reactivity of oxidants formed by iron and copper-catalyzed decomposition of hydrogen peroxide
1
2013
... 双金属催化剂种类繁多,常见的双金属催化剂可分为2类:(1)铁基双金属催化剂.其以铁为基础掺杂其他金属作为催化剂,掺杂金属可加速Fe(Ⅲ)与Fe(Ⅱ)之间的转换,从而提高反应速率.(2)铜基双金属催化剂.铜离子在Fenton反应中体现出耐盐性〔49〕,并有高溶解度〔50〕特性,使得铜在高pH、高盐条件下表现出比铁更高的活性.铜离子参与的Fenton反应与Fe2+/H2O2和Fe3+/H2O2体系的Fenton反应相似〔见式(4)和式(5)〕,并且Cu(Ⅱ)/Cu(Ⅰ)的标准还原电位低于Fe(Ⅲ)/ Fe(Ⅱ)的还原电位,铜基催化剂更容易促进羟基自由基的产生,在热力学上更有利于Fenton系统降解污染物〔51〕.近年来,为了适应复杂的污染环境,铜基双金属催化剂已经逐渐替代铁基双金属催化剂成为研究的重点. ...
Reactivity of novel Ceria-Perovskite composites CeO2-LaMO3(MCu, Fe) in the catalytic wet peroxidative oxidation of the new emergent pollutant 'Bisphenol F': Characterization, kinetic and mechanism studies
1
2017
... 双金属催化剂种类繁多,常见的双金属催化剂可分为2类:(1)铁基双金属催化剂.其以铁为基础掺杂其他金属作为催化剂,掺杂金属可加速Fe(Ⅲ)与Fe(Ⅱ)之间的转换,从而提高反应速率.(2)铜基双金属催化剂.铜离子在Fenton反应中体现出耐盐性〔49〕,并有高溶解度〔50〕特性,使得铜在高pH、高盐条件下表现出比铁更高的活性.铜离子参与的Fenton反应与Fe2+/H2O2和Fe3+/H2O2体系的Fenton反应相似〔见式(4)和式(5)〕,并且Cu(Ⅱ)/Cu(Ⅰ)的标准还原电位低于Fe(Ⅲ)/ Fe(Ⅱ)的还原电位,铜基催化剂更容易促进羟基自由基的产生,在热力学上更有利于Fenton系统降解污染物〔51〕.近年来,为了适应复杂的污染环境,铜基双金属催化剂已经逐渐替代铁基双金属催化剂成为研究的重点. ...
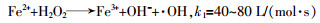









 津公网安备 12010602120337号
津公网安备 12010602120337号